Takemoto, K., Iwanari, H., Tada, H., Suyama, K., Sano, A., Nagai, T., … &
Takahashi, T. (2017). Optical inactivation of synaptic AMPA receptors erases fear memory.
Nature biotechnology, 35(1), 38-47. https://www.nature.com/articles/nbt.3710
“[W]e generated a monoclonal antibody against the extracellular domain of GluA1
and labeled it with the photosensitizer eosin. Upon illumination, this labeled antibody
specifically inactivated GluA1 homomeric CP-AMPA receptors…”, “Exposure of the
hippocampus to green light through an implanted cannula erased the acquired fear memory in the
mice by inactivating synaptic GluA1 homomeric CP-AMPA receptors.”
Mouse hippocampus
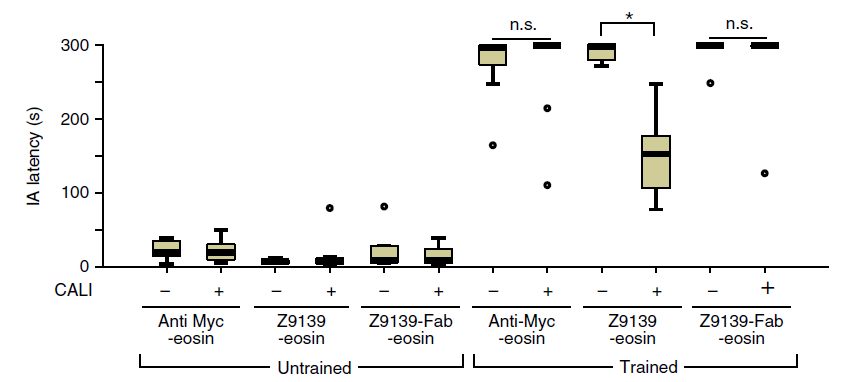
Authors develop an antibody-mediated CALI inactivation technique specifically
targeting ONLY calcium permeable AMPA receptors (GluA1 homomeric CP-AMPA receptors).
Crucially the Ca2+ currents carried by these CP-AMPA are necessary for the protein synthesis
steps needed to convert early LTP to long-lasting LTP. So disrupting these ~1 hour after a
learning event will erase LTP in those synapses encoding that event only while not effecting
other learned events. Authors demonstrated by erasing a fear memory (inhibitory avoidance (IA)
task) in mice.
“Long-term potentiation (LTP) is the mechanism for the activity-dependent strengthening
of synapses that is thought to contribute to learning. GluA1 homomeric receptors are delivered
into synapses at the early phase of LTP, and this delivery is required for LTP maintenance,
presumably by providing Ca2+ through GluA1 homomeric receptors; Ca2+ is critical for protein
synthesis. In this study, we inactivated GluA1 homomeric receptors by in vivo CALI 1 h after
conditioning and erased contextual fear memory. This inactivation could have disrupted Ca2+
influx at the early phase of memory encoding, and caused the continued erasure of fear
memory.”
General expository quote on CALI: “CALI uses a chromophore molecule as a photosensitizer,
which produces short-lived reactive oxygen species, such as singlet oxygen, upon its irradiation
with light. Singlet oxygen elicits a half-radius of photodamage of ~3–4 nm, which is shorter than
the average protein–protein interaction distance (8 nm). Thus, CALI enables a protein to be
inactivated with high spatial resolution. Furthermore, by labeling antibodies with a
photosensitizer, a specific protein on the surface of live cells can be inactivated without
damaging neighboring proteins.
